Fluorescent diamonds
Historical Background:
The history of fluorescent diamonds dates back to European times when the concept of diamond identification did not exist, and the most highly regarded diamonds were "blue-white." This means a diamond with strong blue fluorescence and a color close to colorless to pale yellow. This type of diamond has an attractive "ice" effect and was highly sought after by merchants.
However, it was later discovered that diamonds with solid blue fluorescence were found in hazy opaque diamonds. This discovery caused the price of fluorescent diamonds to drop dramatically in the 1970s when they became known as "milky D" (D color, strong blue fluorescence, low clarity), and even the price of F color diamonds and weakly fluorescent diamonds dropped a few years later.
What is diamond fluorescence?
In general, the diamond characteristics of fluorescent reactions are little known and often misunderstood. Fluorescence is a form of luminescence. A substance is fluorescent if it can stop emitting light when the light source disappears. Diamond Fluorescence (Diamond Fluorescence) refers to the visible light emitted under the excitation of intense UV light, figuratively speaking, like the usual security markings on banknotes, the kind of light that can only be seen under a money detector or bright light.
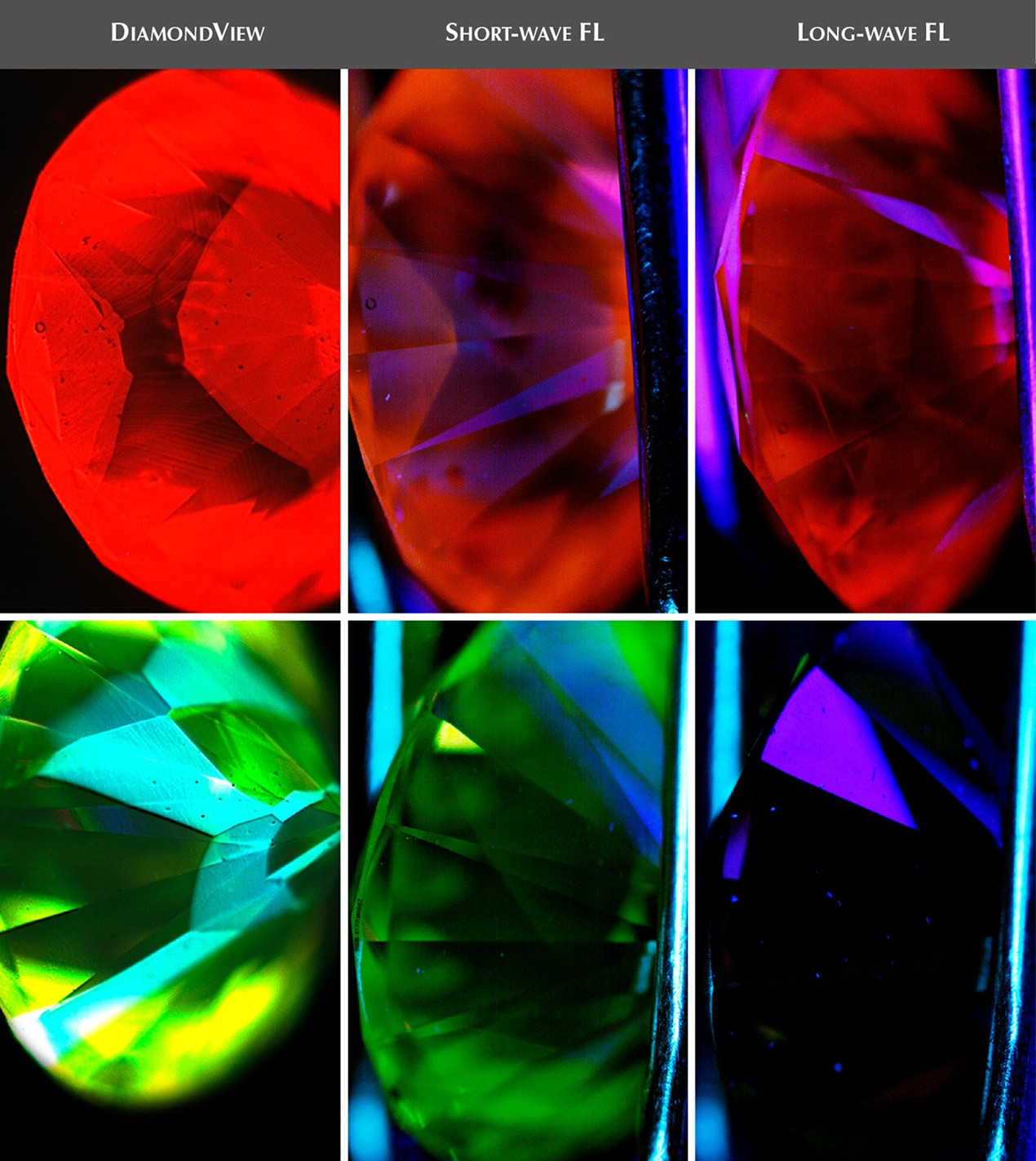
Why do diamonds fluoresce?
Diamonds are carbon and must be formed roughly 75 to 125 miles deep in the earth's surface, usually where volcanoes or ancient volcanic sites are located. If trace amounts of the mineral boron are mixed with the diamond during its formation, it will have a fluorescent effect.
Does every diamond have fluorescence?
Not all diamonds fluoresce. According to GIA's professional statistics, only about 25% to 35% of diamonds show some fluorescence under standard long-wave UV light. And only 10% of that fluorescence affects the appearance of the diamond. Therefore, the diamonds you buy do not necessarily fluoresce.
Under what circumstances can I see the fluorescence reaction of a diamond?
Diamonds fluoresce only when exposed to invisible ultraviolet light or other sources of radiation with high energy (X-rays and lasers).
The fluorescence of diamonds is likely to be seen in the intense sunlight people encounter in their daily lives, in tanning beds, dance bars, or other places where harsh lighting is used.
However, once a diamond is removed from these light sources, it will stop fluorescing. LED or incandescent lights in public life do not cause diamonds to fluoresce.
The use of specialized instruments for detecting fluorescence, such as those used in GIA laboratories to detect fluorescence, will strictly control the light source and environment and follow professional standards to assess the fluorescence response of each diamond to ensure that the appraisal report is accurate and official.
GIA diamond fluorescence reaction grading effect?
In addition to the international "4C's" to assess the quality of a diamond, fluorescence is another important supporting criterion for diamonds. The GIA considers fluorescence as an identifying feature that helps distinguish between different types of diamonds. The intensity of fluorescence can be classified into the following five levels:

*None (no fluorescence)
*Faint (faint fluorescence)
*Medium (medium fluorescence)
*Strong (strong fluorescence)
*Very Strong(very strong fluorescence)
The effect of fluorescence reaction on diamonds?
The fluorescence response of a diamond can be good or bad, a double-edged sword that can either improve the color of a diamond or may make it look hazier. Based on the consumer's visual perception, the problem can be dissected into three areas: first, the color of the diamond; second, the transparency of the diamond. And third, the sparkle of the diamond.
Color: Blue fluorescence can make a colorless or yellowish diamond appear whiter and closer to colorless transparency, thus improving the appearance of the diamond, so diamond fluorescence is not entirely harmful. It is essential to compare the strength of its fluorescence with what color grade the fluorescence appears on the diamond. For example, a diamond with a Medium fluorescence grade will appear whiter.

An important factor affecting colored diamonds is the vibrancy of their color. In the right light, the fluorescence emitted by particular colored diamonds will blend with their color. The self-fluorescence will give this diamond an incredible color change, giving the viewer a visual impact. A fancy-colored diamond with complementary fluorescence can enhance the vibrancy of its color. For example, a yellow diamond that emits yellow fluorescence will be more vibrant when exposed to sunlight, ultraviolet light, or other intense light.

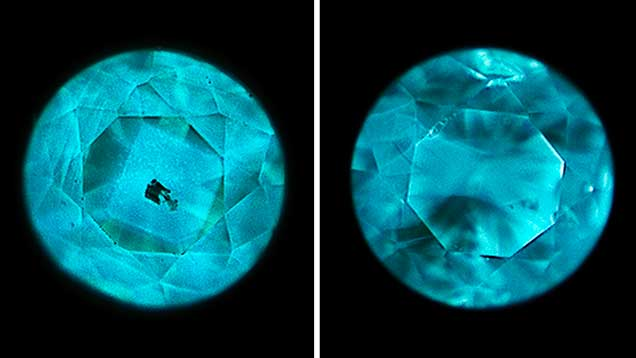
Transparency: However, diamond fluorescence can cause D-G color diamonds with high color grades to appear misty or oily, making the diamond hazy and losing its transparency and clarity. However, most diamonds with solid fluorescence do not show this greasy appearance, and only 0.2% of GIA's firmly fluorescent diamonds show this effect. The price of such diamonds will be lower than those that do not fluoresce.

Sparkle: refers to the brightness, fire, and flash that a diamond exhibits. However, the effect of fluorescence on these three is minimal and does not affect the visual impact of the diamond. Studies have shown that fluorescence does not affect the beauty of a diamond and that the sparkle that a diamond gives people depends mainly on the cut of the diamond and has nothing to do with the fluorescence of the diamond.

Misconceptions about the fluorescence response of diamonds
#Fluorescence detracts from the durability of diamonds
A diamond that fluoresces under standard ultraviolet light under conditions of structural integrity is no different from a diamond that does not fluoresce, and a diamond with a fluorescent reaction does not inherently diminish its durability of the diamond.
#Diamonds with fluorescence reaction can only be called [naturally mined diamonds]
This is not true. Not all natural diamonds fluoresce; some lab-grown diamonds also fluoresce under intense UV light. Synthetic zirconia, a material used to imitate diamonds, can also fluoresce. Although the fluorescence of natural and synthetic diamonds varies in intensity, color, and pattern shape, some similarities cannot be ruled out. Therefore, the presence or absence of fluorescence is not a criterion for determining the authenticity of a diamond.
#Fluorescent reaction is harmful to the body, a kind of radiation
The fluorescence of diamonds is a natural property, the reason why fluorescence occurs is because of the optical phenomenon that the nitrogen or boron elements inside the diamond are excited under intense ultraviolet light, and there is no radioactivity, no radiation, no negative impact on human health, and can be worn without worry. Some diamonds are even believed to promote health.
#Fluorescence reaction affects the color grade of the diamond
When grading the color of a diamond, GIA follows strict criteria in grading the diamond under strictly controlled viewing environments and lighting conditions to minimize the effect of fluorescence on the diamond. Therefore fluorescence reaction does not affect the color grade of a diamond. However, when a diamond is observed under specific lighting conditions, the fluorescence intensity can affect the diamond's color as observed by the human eye. Therefore, when assessing the quality of a diamond's appearance, it is better to consider each diamond individually.
#Diamonds only show blue fluorescence
Diamonds can fluoresce in a variety of colors. These include orange, yellow, red, white, and green. Differences in the atomic structure of diamonds, such as different numbers of nitrogen atoms, can cause diamonds to fluoresce in different colors. However, by far, blue is the most common diamond fluorescence color.
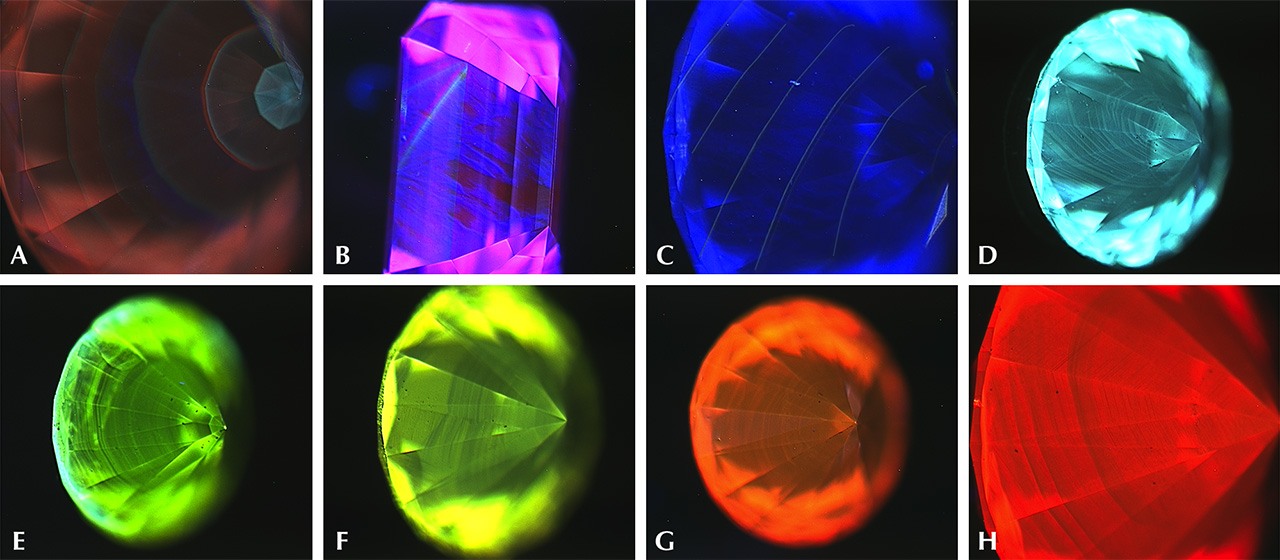
#Strong blue diamond fluorescence is bad
The intensity of fluorescence emitted by a diamond may have a positive impact on the diamond, such as in the GIA's D to Z color scale, lower grade (I to N) yellow diamonds, which contain moderately blue solid fluorescence, may offset some of the yellow, making the diamond appear more pure and sparkling, giving the diamond's making the diamond look and color better than the corresponding diamond in the grading system.
Can you tell if a diamond has fluorescence?
To address this issue, the GIA Diamond Certification Laboratory in the United States has researched the subject. The researchers used four groups of diamonds of different color grades (colors E, G, I, and K), with six diamonds in each group, each of which had essentially the same quality, except for the difference in fluorescence intensity of the diamonds. The diamonds were then judged by different people (trained diamond graders, trade professionals, and general observers) who observed the diamonds with the naked eye.
The study's results showed that for the average observer, who represents the jewelry-buying public, it was not possible to observe with the naked eye the difference in the intensity of fluorescence of each group of diamonds. Even experienced professionals have a great deal of trouble distinguishing them. Thus, we can see that the effect of a diamond's fluorescence on colorless and nearly colorless diamonds (color grades from D to J) can be considered minimal. Only when the fluorescence is robust is it seen to have some effect on the diamond.
How to choose the fluorescence level of a diamond?
If you are looking for perfection, when choosing a colorless diamond with a color grade of D-F, it is best to choose a diamond with a fluorescence grade of "None" or "Faint" to avoid a milky white or oily haze phenomenon that reduces the diamond's transparency will be diminished, and the visual effect will be affected.
If you want to buy a very high-value diamond with a very high color grade and imperfections that are not visible to the naked eye, a diamond with a colorless G-I grade and a fluorescence grade of "Medium Blue" or "Strong Blue "Is when the blue fluorescence of the diamond enhances the whiteness of the diamond and makes it appear more colorless and pure, making it highly cost-effective.
If your budget is limited, choose a diamond with a good cut, Si1-Si2 clarity, and a fluorescence level of "Very Strong Blue," which, when viewed with the naked eye, is no less impressive, and you are likely to like the unique effect created by this fluorescence even more, very characteristic.

What effect does fluorescence have on the price of a diamond?
Jewelry professionals do not believe that fluorescence increases or decrease a diamond's value. Still, firmly fluorescent diamonds have been less expensive than non-fluorescent diamonds. Typically, for higher color grades (D to H), non-fluorescent diamonds can cost 10-30% more than diamonds with robust blue fluorescence because fluorescence can make a diamond look faint or cloudy, affecting the clarity of the diamond. However, for diamonds with lower color grades (I to N), diamonds with solid fluorescence are 5% more expensive than diamonds without fluorescence. This may be because people feel that blue fluorescence can mask some diamonds' undesirable light yellow color.
Are fluorescent diamonds worth buying?
For consumers, the most important thing is to buy the diamond they like. In summary, fluorescence can significantly enhance the color and appearance of a diamond under certain conditions, and only in rare cases will it appear milky, oily, or hazy, so it should not be considered a disadvantage. The world's most famous blue diamond, the Hope diamond (hope), is a diamond with a strong fluorescence (Very Strong Blue). We recommend that consumers purchase a diamond by looking at it from different angles and under other lights. They can consult a diamond expert before purchasing to ensure that the diamond they choose is the one they love.
Leave A Message
The first thing we do is meet with our clients and talk through their goals for a future project.
During this meeting, feel free to communicate your ideas and ask lots of questions.
Copyright ©2025 Wuzhou Tianyu Gems Co., Ltd - All Rights Reserved.